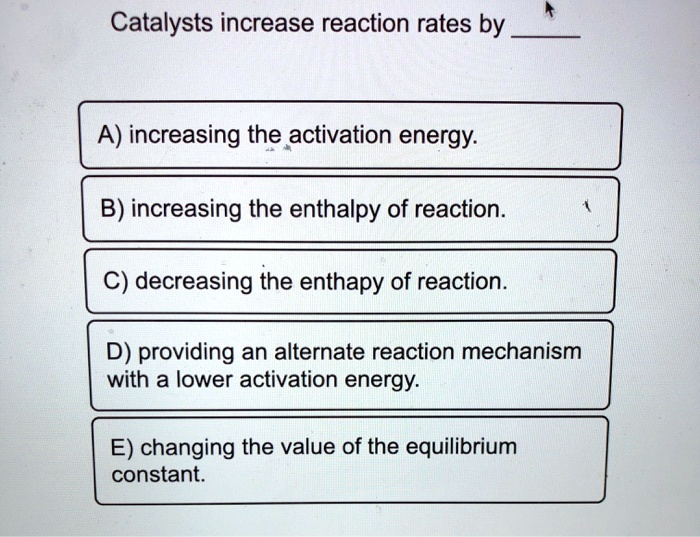
Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A . It assumes that you are already familiar with basic ideas about the collision. The speed at which it occurs, without itself being part of the reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and. each kind of catalyst facilitates a different pathway with its own activation energy. this page explains how adding a catalyst affects the rate of a reaction. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Because the rate is an exponential function of e a (arrhenius equation),. a catalyst is a compound or element that increases the rate of a chemical reaction, e.g. reaction diagrams for a chemical process with and without a catalyst are shown below. Investigate the rate of reaction by colour change.
from www.numerade.com
catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. It assumes familiarity with basic concepts in the collision theory of reaction rates, and. Because the rate is an exponential function of e a (arrhenius equation),. this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision. each kind of catalyst facilitates a different pathway with its own activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below. a catalyst is a compound or element that increases the rate of a chemical reaction, e.g. The speed at which it occurs, without itself being part of the reaction. Investigate the rate of reaction by colour change.
SOLVED Catalysts increase reaction rates by A) increasing the
Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A Because the rate is an exponential function of e a (arrhenius equation),. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below. Investigate the rate of reaction by colour change. Because the rate is an exponential function of e a (arrhenius equation),. a catalyst is a compound or element that increases the rate of a chemical reaction, e.g. this page explains how adding a catalyst affects the rate of a reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and. The speed at which it occurs, without itself being part of the reaction. It assumes that you are already familiar with basic ideas about the collision. each kind of catalyst facilitates a different pathway with its own activation energy.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A this page describes and explains the way that adding a catalyst affects the rate of a reaction. Because the rate is an exponential function of e a (arrhenius equation),. Investigate the rate of reaction by colour change. a catalyst is a compound or element that increases the rate of a chemical reaction, e.g. It assumes that you are. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A a catalyst is a compound or element that increases the rate of a chemical reaction, e.g. It assumes that you are already familiar with basic ideas about the collision. Because the rate is an exponential function of e a (arrhenius equation),. this page explains how adding a catalyst affects the rate of a reaction. catalysts increase the. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A Investigate the rate of reaction by colour change. each kind of catalyst facilitates a different pathway with its own activation energy. It assumes familiarity with basic concepts in the collision theory of reaction rates, and. Because the rate is an exponential function of e a (arrhenius equation),. this page explains how adding a catalyst affects the rate of. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From slideplayer.com
Chemical Reactions and Collision Theory ppt download Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A this page describes and explains the way that adding a catalyst affects the rate of a reaction. Investigate the rate of reaction by colour change. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The speed at which it occurs, without itself being part of the reaction. It assumes. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A It assumes that you are already familiar with basic ideas about the collision. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts increase the rate of a reaction by lowering. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A each kind of catalyst facilitates a different pathway with its own activation energy. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. this page explains how adding a catalyst affects the rate of a reaction. The speed at which it occurs, without itself being part of the. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A It assumes that you are already familiar with basic ideas about the collision. each kind of catalyst facilitates a different pathway with its own activation energy. this page describes and explains the way that adding a catalyst affects the rate of a reaction. this page explains how adding a catalyst affects the rate of a reaction. It. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From www.numerade.com
SOLVEDA catalyst increases rate of reaction by (a) decreasing enthalpy Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A The speed at which it occurs, without itself being part of the reaction. reaction diagrams for a chemical process with and without a catalyst are shown below. Because the rate is an exponential function of e a (arrhenius equation),. a catalyst is a compound or element that increases the rate of a chemical reaction, e.g. Investigate the rate. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A Because the rate is an exponential function of e a (arrhenius equation),. each kind of catalyst facilitates a different pathway with its own activation energy. this page explains how adding a catalyst affects the rate of a reaction. catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the.. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From www.savemyexams.com
Catalysis OCR A Level Chemistry Revision Notes 2017 Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A each kind of catalyst facilitates a different pathway with its own activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below. this page explains how adding a catalyst affects the rate of a reaction. a catalyst is a compound or element that increases the rate of a chemical reaction, e.g.. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From slideplayer.com
TOPIC 11 AND EQUILIBRIUM ppt download Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A It assumes familiarity with basic concepts in the collision theory of reaction rates, and. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. this page explains how adding a catalyst affects. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From exohgcpop.blob.core.windows.net
A Catalyst Increases The Rate Of Reaction As It at Gladys McCoy blog Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. each kind of catalyst facilitates a different pathway with its own activation energy. Because the rate is an exponential function of e a (arrhenius equation),. this page describes and explains the way that adding a catalyst affects the. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. each kind. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From www.numerade.com
SOLVED Draw a reaction coordinate diagram for the reversible reaction Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A each kind of catalyst facilitates a different pathway with its own activation energy. this page describes and explains the way that adding a catalyst affects the rate of a reaction. Because the rate is an exponential function of e a (arrhenius equation),. this page explains how adding a catalyst affects the rate of a reaction. a. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From millerdidettioners.blogspot.com
How Does Particle Size Affect Reaction Rate Miller Didettioners Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Investigate the rate of reaction by colour change. reaction diagrams for a chemical process with and without a catalyst are. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From studybeglerbegs.z4.web.core.windows.net
How Do Enzymes Catalyze Reactions Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Investigate the rate of reaction by colour change. this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and. . Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A catalysts increase the rate of a reaction by lowering the activation energy by providing an alternative pathway for the. The speed at which it occurs, without itself being part of the reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. a catalyst is a compound or element that. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.
From www.numerade.com
SOLVED Catalysts increase reaction rates by A) increasing the Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A reaction diagrams for a chemical process with and without a catalyst are shown below. a catalyst is a compound or element that increases the rate of a chemical reaction, e.g. It assumes that you are already familiar with basic ideas about the collision. this page describes and explains the way that adding a catalyst affects the rate. Catalysts Increase The Rate Of Reaction By Providing A Reaction Pathway With A.